Electron Probe Microanalysis
This information cannot be obtained by x-ray diffraction or optical microscopy. The electron microprobe and high-resolution SEM are essential for process mineralogy problem solving; we have used them to:
- Locate submicroscopic gold in sulfides and other minerals;
- Determine the disposition of heavy metals such as uranium, lead, chromium, cadmium, etc., at low levels in contaminated soils or hazardous wastes;
- Analyze slags or furnace and boiler deposits to determine the cause and mechanism of detrimental scale formation;
- Locate valuable or hazardous elements occurring at low levels or as substituting ions in minerals for which they are not an essential constituent.
The following example illustrates a problem that was solved with the electron microprobe. For some copper deposits, the extraction obtained by acid leaching is frequently not as good as expected. One of the primary reasons is that copper sometimes occurs associated with iron oxides in acid-insoluble form. This association is illustrated below by means of backscattered electron and x-ray images of the elements of interest.
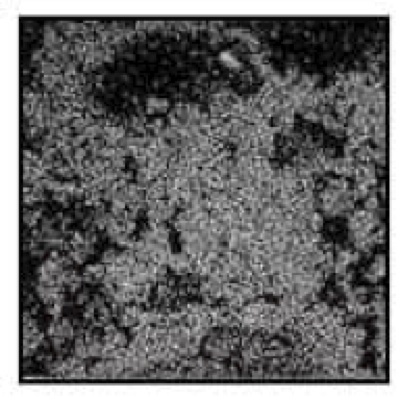
X-Ray Map Showing
Copper Distribution in the
Same Goethite Grain
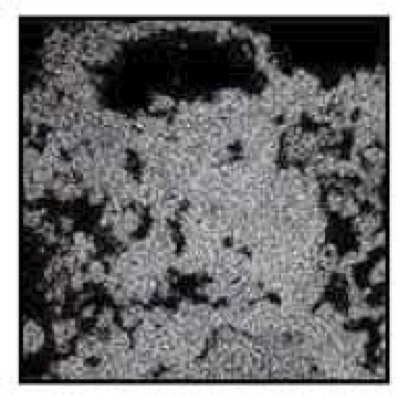
X-Ray Map Showing Variable
Concentrations of Iron in the
Same Goethite Grain

Backscattered Electron
Image Showing Heavy
Element Concentration
This image shows a goethite (FeOOH) particle in an oxide copper ore. Areas of greater brightness correspond to heavier elements; darker areas correspond to lighter elements. The structure of the grain is typical of goethite derived from the oxidation of sulfides.
The spatial correlation between copper and iron occurrence illustrates a submicroscopic, perhaps structural, association of copper with the hydrated iron oxide. This copper would not be recoverable by conventional leaching. In a standard analysis for acid-soluble copper and sulfide copper, the copper associated with hydrated iron oxides is frequently misinterpreted as sulfide copper.

Service Applications
Related Capabilities
Contact Hazen
Main (303) 279 4501
Fax (303) 278 1528
E-mail / Directions
Submit RFP
